Do I Need IRB Review?
File a NHSR Determination
USU’s IRB does not give unofficial determinations regarding whether a project requires IRB review. The only determination available is via the Non-Human Subjects Research (NHSR) Determination form in Kuali Protocols. This very brief form ensures that the IRB has all of the information necessary to render an accurate determination about whether any given project requires IRB review.
The IRB makes this form available for the convenience of USU’s researchers, so that they can obtain official paperwork regarding the need for IRB review, which can be shared with sponsors, department and college leadership, collaborators at other institutions, journal editors, etc. There may be times where a researcher is required to complete one (for example, in the course of a noncompliance inquiry or in order to verify a regulatory status prior to submitting a proposal or setting up an award), but on the whole, submitting an NHSR is voluntary. You can view the form here, in its entirety. (Most submissions are shorter; this is the longest possible form based on the logic built into it.) Additional help text is available in the form itself – that text does not show up on the document provided here.
How does the IRB decide?
Two elements must be present for IRB oversight to be necessary:
- The project must be “research” as it is defined in 45 CFR 46 (the “Common Rule”).
- Research is defined as a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.
- A systematic investigation is a process that involves the formulation of a hypothesis, exploration of a theme, or establishment of research questions, and the collection and/or analysis of data that will lead to a conclusion that either proves or disproves the hypothesis, addresses the themes, or that answers the research question.
- Generalizable knowledge is knowledge that is “expressed in theories, principles, and statements of relationships” that can be widely applied to our experiences and is usually created to share with other people, such as through presentations and publications.
- The project must involve a “human subject” as defined in 45 CFR 46.
- A human subject is defined as a living individual about whom an investigator conducting research obtains
- data or specimens through intervention or interaction with the individual; OR
- identifiable private information or specimens.
- A human subject is defined as a living individual about whom an investigator conducting research obtains
A project that involves “research” with a “human subject” must have IRB approval or exemption in place before it can begin.
There are certain activities that are excluded from the definition of research by their very nature, including some journalism and oral history projects, certain public health surveillance activities, certain criminal justice activities, some biographical and historical work, and operational activities in support of intelligence/homeland security/national security missions. A certain proposal type (Other Sponsored Activity, Training & Instruction, etc.) in the Sponsored Programs module does not in and of itself determine whether a project is "research" under the 45 CFR 46 definition; there is considerable crossover between "research" with a "human subject" and proposal/award types other than "Research: Basic."
How do I file an NHSR Form?
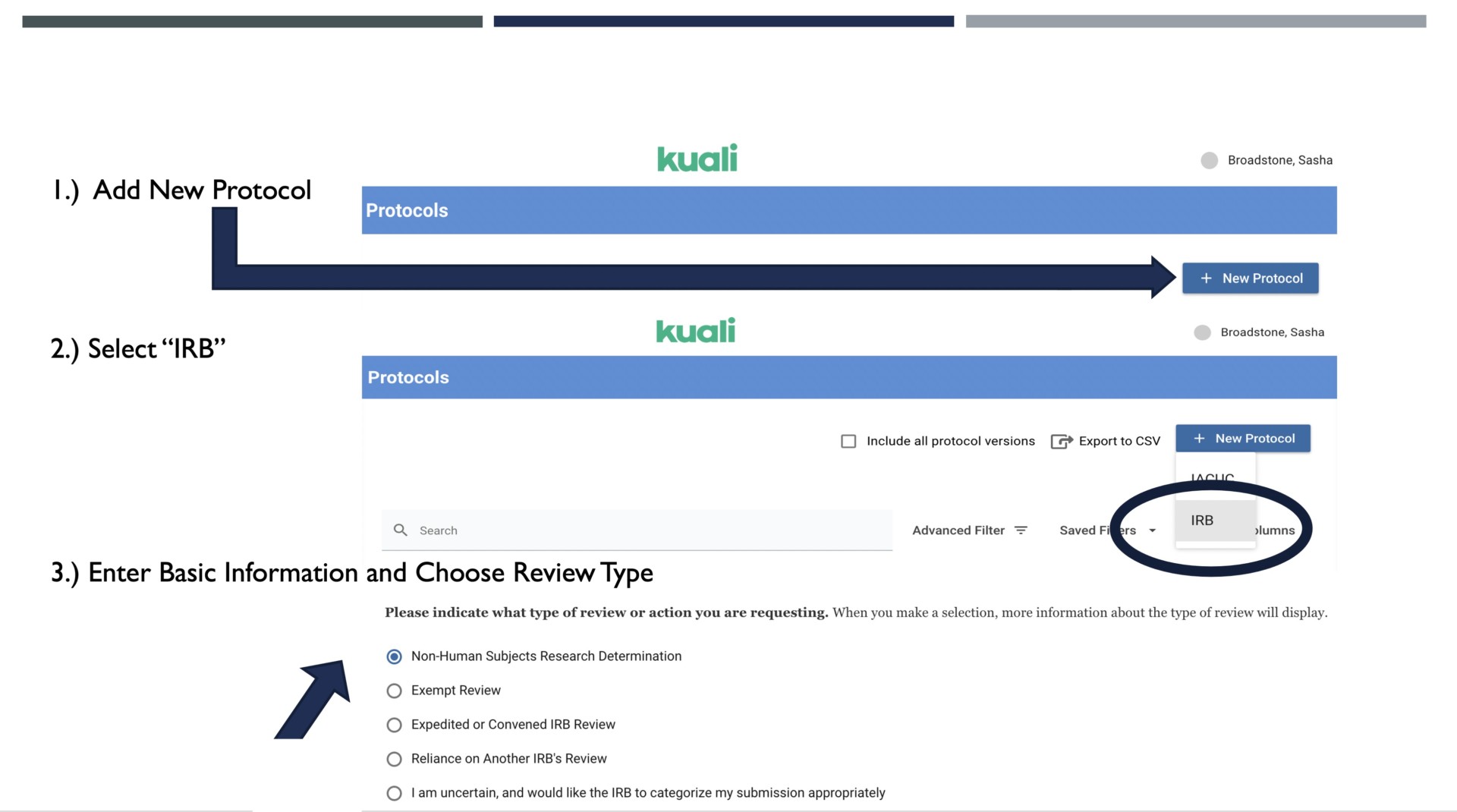 In Kuali Protocols, select “New Protocol” and then "IRB." You will provide some basic information about the study (like a title, funding source, start date, etc.) and then, in the question that asks what type of review you are seeking, select “Non-Human Subjects Research Determination.” Unlike substantive protocol submissions (Exempt, Expedited, Convened IRB protocols), any USU affiliate can submit a NHSR Determination, and CITI Training is not prerequisite to submission. Please do note, though, that students are typically not in the Kuali system. If you receive an "Unauthorized" or "404" error, please contact Dan Perry to request access to Kuali Protocols.
In Kuali Protocols, select “New Protocol” and then "IRB." You will provide some basic information about the study (like a title, funding source, start date, etc.) and then, in the question that asks what type of review you are seeking, select “Non-Human Subjects Research Determination.” Unlike substantive protocol submissions (Exempt, Expedited, Convened IRB protocols), any USU affiliate can submit a NHSR Determination, and CITI Training is not prerequisite to submission. Please do note, though, that students are typically not in the Kuali system. If you receive an "Unauthorized" or "404" error, please contact Dan Perry to request access to Kuali Protocols.
View the NHSR Determination Template.

